LSU Research Bites: A Superbug-Inspired Platform Targets Cancer Cells While Sparing Healthy Tissue
January 16, 2026
Any biologist knows to keep bacteria far away from mammalian cell cultures in the lab, to avoid a lot of headaches from contaminated cell culture samples and experiments.
That is, unless you are Dr. Chen Chen and you’ve had the “crazy idea” of harnessing bacterial toxins against human cancer cells. In a new study published in Biochemical and Biophysical Research Communications, Chen describes a novel anticancer platform that tames bacterial toxins into homing devices for cancer cells undergoing uncontrolled growth.
Chen, an assistant professor in the Department of Biological Sciences in the LSU College of Science, studies Staphylococcus aureus (staph), a bacterium notorious as a “superbug” and the most common cause of hospital-acquired infection.
Staph is actually an opportunistic bacterium that lives on the skin of one in three people without harm — it only causes severe infection in people who are for some reason immune compromised.
Problem: Existing cancer immunotoxin drugs that target cancer cells with toxins often derived from bacteria suffer from low binding specificity and affinity, causing damage to healthy cells.
Solution: Staphylococcus aureus expresses a protein that effectively targets the cell surface glycan SLeX, which is present on immune cells but also overexpressed on cancer cells. Fusing this targeting protein with a bacterial toxin produces a novel anticancer platform for targeting and killing cancer cells.
Impact: The resulting anticancer platform targets a common cancer marker regardless of the type of cancer, while displaying low toxicity in non-cancer cells.
Chen is a leading expert on the specialized proteins and toxins that Staphylococcus aureus uses to manipulate and exploit weaknesses in the immune system. Among these are 14 staphylococcal superantigen-like (SSL) proteins that suppress our innate immune cells. Chen has extensively studied one of these proteins, called SSL11, that targets immune cells like neutrophils, making them “sticky,” slow, and ineffective at fighting off infection.
But in studying SSL11, Chen noticed something interesting. This protein targets the glycan Sialyl Lewis X (SLeX), a sugar structure common not only on the surface of human immune cells, but also on cancer cells. Glycans help immune cells move around and infiltrate sites of infection or inflammation. Many cancer cells also develop a ton of glycans on their surfaces, helping metastatic cancer cells spread to places they shouldn’t.
“I had this crazy idea,” Chen said. “Since SSL11 binds to glycan cancer markers, maybe we could use this protein for good, to target and deliver killing components into cancer cells.” The fact that SSL11 binds the glycan SLeX to inhibit cell movement makes it an appealing delivery platform against cancers that overexpress SLeX.
The Perfect Laboratory Set-up

“Our study will provide new ideas for our battle against cancer, especially those prone to metastasis.” – Dr. Chen Chen, LSU
As Chen gives a tour of her lab, she points out how lucky she was to be assigned three separate rooms on the 6th floor of the Life Sciences Building.
One room is reserved for bacterial cultures, including the notorious Staphylococcus aureus that Chen studies. The next is a sterile, general wet lab space, and the last unconnected room is dedicated to mammalian cell culture and microscopy. This setup allows Chen to effectively study staph in one space and the impact of staph-derived proteins and toxins on immune and cancer cells in another.
In her recently published study, Chen’s lab adapted a potent bacterial toxin from the bacteria Pseudomonas aeruginosa that kills cells by interfering with protein synthesis.
While bacterial endotoxins have previously been used in cancer treatments by pairing them with antibodies that target cancer cells, these engineered cancer-killing proteins often exhibit low targeting and binding abilities. Chen proposed fusing Pseudomonas endotoxin (PE) to SSL11 to enable effective targeting, binding, and killing of cancer cells.
A Potent Homing Device
Chen’s lab found that their engineered fusion protein successfully bound to lung cancer cells and delivered the endotoxin into the cells. Once inside, the entotoxin began to essentially paralyze and kill the cancer cells.
Neither SSL11 nor the endotoxin, when used alone and unfused, was able to enter and kill cancer cells.
The fusion protein also did not kill non-cancer cells—it was even less toxic than other endotoxin-based cancer drugs—and was not overly toxic to immune cells with surface glycans. SSL11 binds SLeX with high specificity, outperforming many glycan-targeting antibodies that suffer from low affinity and off-target effects.
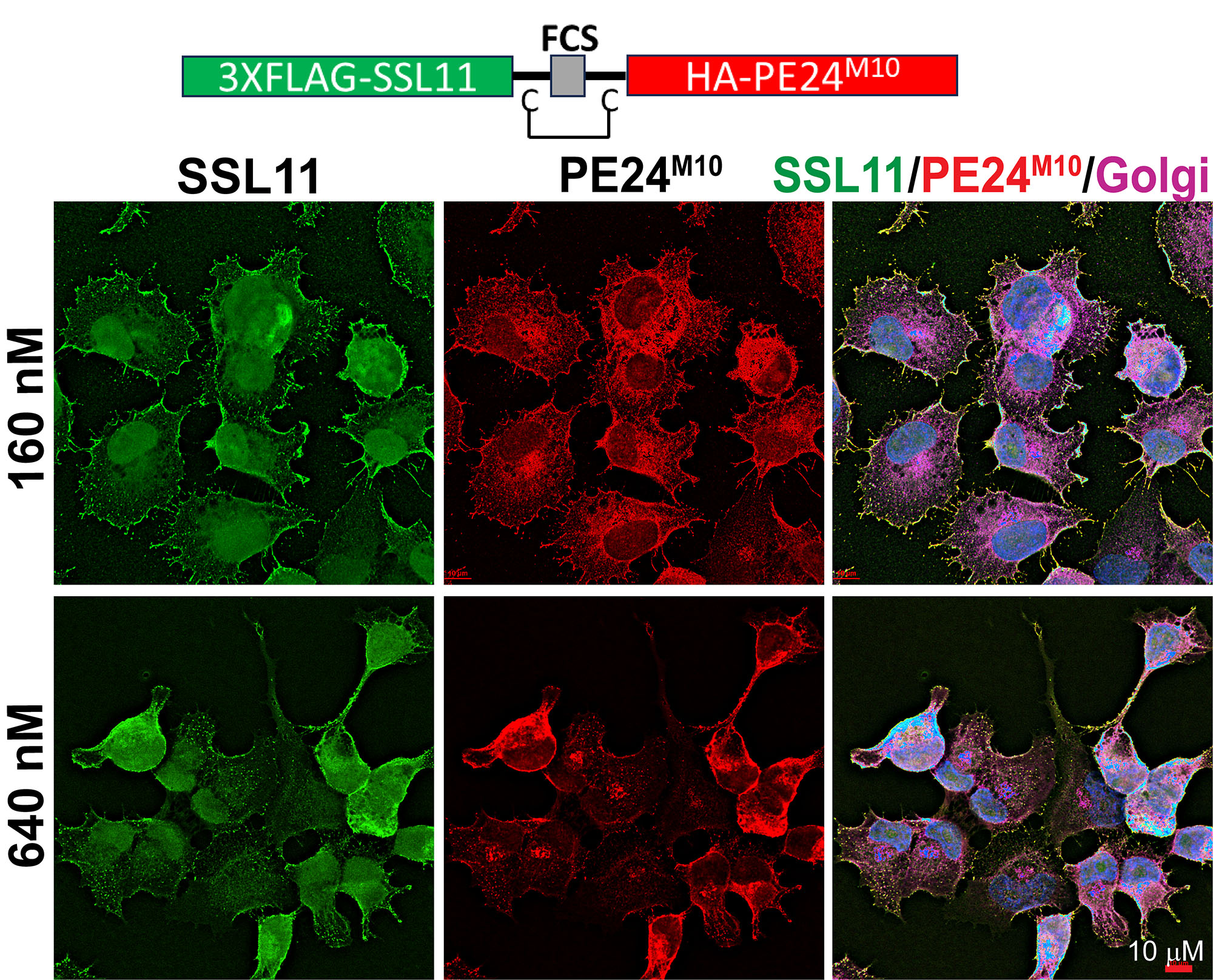
Cells treated with 640 nM of SSL11-PE began to round up, suggesting that it started to induce cell death.
“These results are really promising,” Chen said.
The next step is to test this fusion protein in vivo, for example, in mouse cancer models. While further research is needed, pairing a cancer “address label” protein from staph bacteria with a bacterial toxin has significant potential as a new approach to target and treat hypoxic, metastatic cancers. SSL11 can potentially be paired with other toxin domains or engineered for even greater specificity.
Chen was an MD before she was an academic researcher. In medical school, she was troubled that many of the mechanisms underlying the diseases she was learning to treat remained unknown. She decided to uncover disease mechanisms rather than merely treat symptoms and embarked on an academic research career. Today, she is passionate about conducting translational research that has clinical applications.
Chen’s work has resulted in a publication, a patent (PCT/US24/48990; WO2025/072772), and a $379K NIH R21 federal grant (2024-2026).
Read the study: Yang, C., & Chen, C. (2025). Taming bacterial toxins into an anticancer platform. Biochemical and biophysical research communications, 152976.
Next Step
LSU's Scholarship First Agenda is helping achieve health, prosperity, and security for Louisiana and the world.


